Product
Product Name: Linagliptin
Structure:
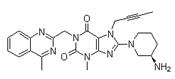
CAS NO: 668270-12-0
Specification: In-house
Documents: MSDS;Tech Package
Usage: Linagliptin belongs to a class of drugs called DPP-4 inhibitors,diabetes mellitus type 2.
Supplementary information: Once-daily linagliptin was approved by the U.S. Food and Drug Administration (FDA) on 2 May 2011 for treatment of type 2 diabetes. It is being marketed by Boehringer Ingelheim and Lilly.
Structure:

CAS NO: 668270-12-0
Specification: In-house
Documents: MSDS;Tech Package
Usage: Linagliptin belongs to a class of drugs called DPP-4 inhibitors,diabetes mellitus type 2.
Supplementary information: Once-daily linagliptin was approved by the U.S. Food and Drug Administration (FDA) on 2 May 2011 for treatment of type 2 diabetes. It is being marketed by Boehringer Ingelheim and Lilly.



