Product
Product Name: Ticagrelor
Structure:
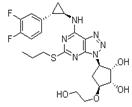
CAS NO: 274693-27-5
Specification: In-house
Documents: MSDS;Tech Package
Usage: prevention of thrombotic events (for example stroke or heart attack) in people with acute coronary syndrome or myocardial infarction with ST elevation
Supplementary information: approved for use in the European Union by the European Commission on December 3, 2010. The drug was approved by the US Food and Drug Administration on July 20, 2011.
Structure:

CAS NO: 274693-27-5
Specification: In-house
Documents: MSDS;Tech Package
Usage: prevention of thrombotic events (for example stroke or heart attack) in people with acute coronary syndrome or myocardial infarction with ST elevation
Supplementary information: approved for use in the European Union by the European Commission on December 3, 2010. The drug was approved by the US Food and Drug Administration on July 20, 2011.



